4. How to Achieve Fast Elution
Slow elution of your target compound extends analysis time and results in low-throughput. Here, we introduce some methods to shorten analysis time mainly with ODS columns.
4-1. Increasing the Elution Strength of the Mobile Phase
In reversed phase mode, analytes are less retained as the organic content in the mobile phase increases. If your analytes elute slowly even with a 100% organic solvent as the mobile phase, stronger solvents; for example, tetrahydrofuran (TFA) or 2-propanol (IPA) instead of methanol or acetonitrile, may decrease the retention.
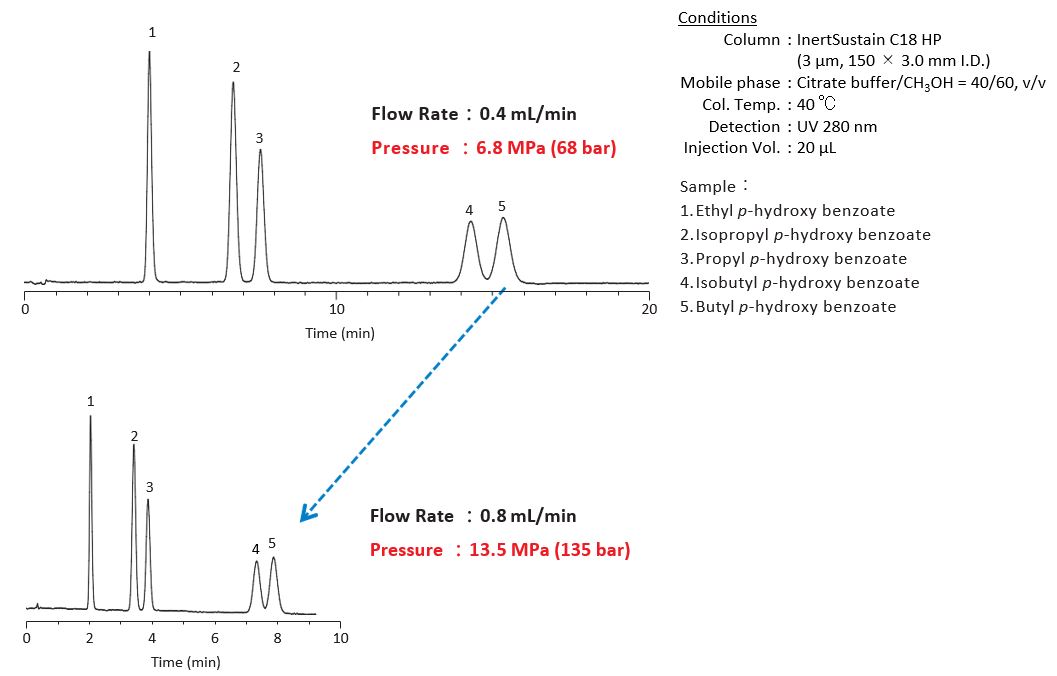
4-2. Increasing the Flow Rate
Increasing the flow rate results in faster elution and short analysis time. In this case, note that the pressure gets higher and the flow rate can be out of its optimal. (See Figure 1-4 on p. 207 for the maximum operating pressure on the column and p. 193 for the optimal flow rate.)
4-3. Decreasing the Carbon Load or the Surface Area of the Packing Material
Decreasing the carbon load or the surface area of the packing material weaken retention. See details in Section 3-3.
4-4. Using C8 and C4 columns
C8 and C4 columns possess shorter alkyl chains as the functional groups than ODS columns, resulting in weaker hydrophobic retention.
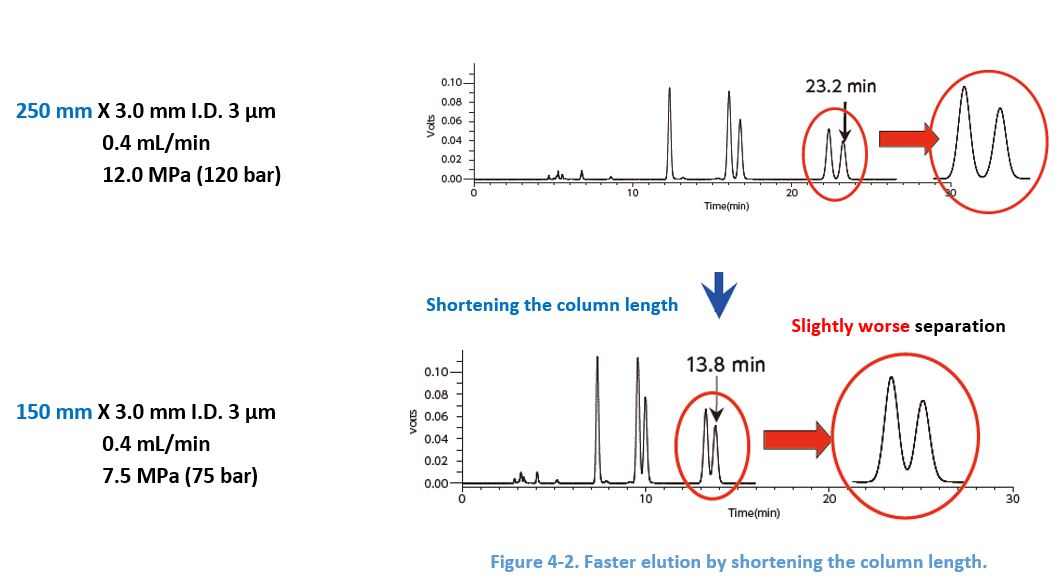
4-5. Shortening the Column Length
For a given flow rate, elution becomes faster when the column length shortened because of the smaller column volume. However, note the separation is worsened because there are less sites for the interaction.
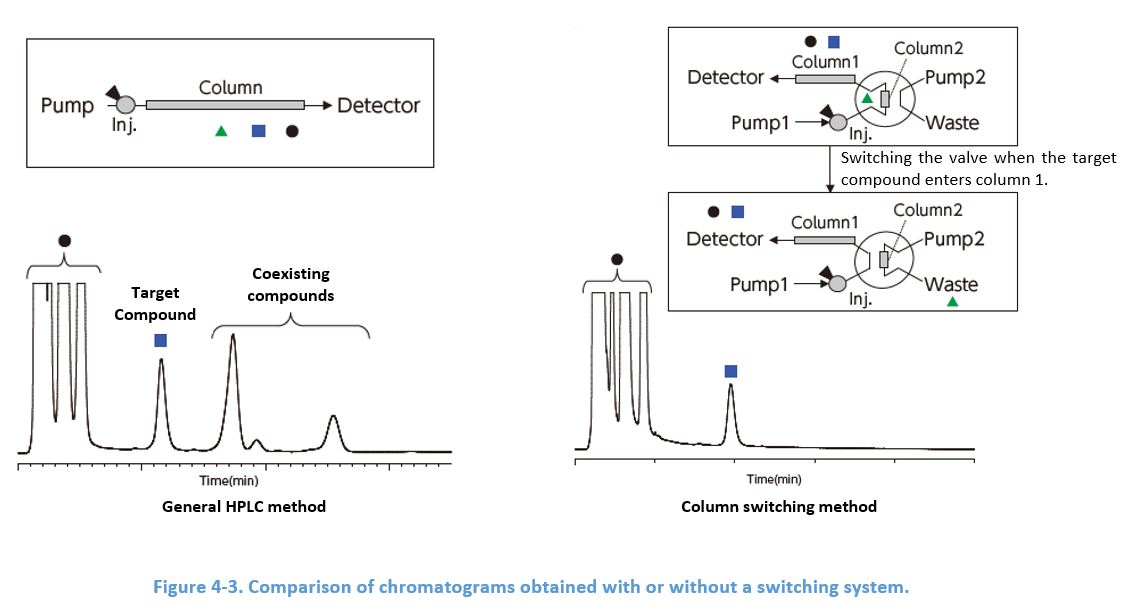
4-6. Building a Switching System Using 2 Columns
Attaching a short pre-column packed with the same material as the analytical column prevents impurities expected to elute later than the target compound from entering the analytical column. Additional pump and a switching valve are needed, however, this method is quite effective especially to routine analysis suffering from long analysis time because of these impurities.
Adsorption of the target compound on inner walls of sample vessels like vials decreases the sample concentration injected to the system. As a consequence, the amount of the target compound reaching the detector is reduced. For example, silanol groups in glass adsorb basic compounds, while plastics adsorb hydrophobic compounds. Choose a proper material for your sample vessels, depending on your target compound.