1. How to Use Buffers
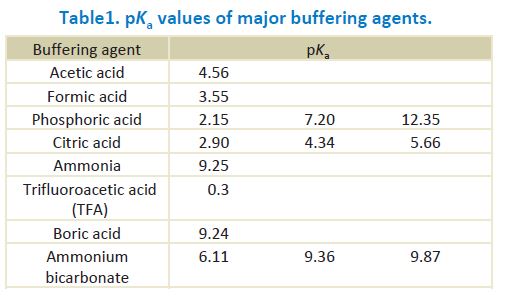
Table 1 shows pKa values of frequently used buffers in reversed phase HPLC. Ka stands for an acid dissociation constant and pKa is its negative logarithm. The buffer capacity becomes maximum when the pH of the buffer is its pKa.
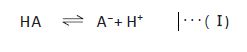
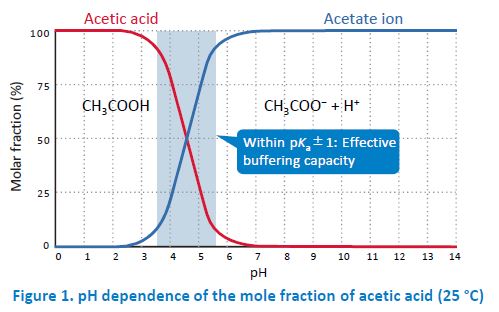
In general, acetate buffers are prepared by mixing acetic acid and sodium acetate. However, when a volatile buffer is needed (e.g. for LC/MS(/MS)), ammonium acetate is used instead of nonvolatile sodium acetate. When an acid or a base is added to a buffer, the pH change is suppressed by a shift of the equilibrium (I) in the direction of diminishing the increase in the concentration of the added compound. This buffering capacity is highest when the pH is within pKa±1. For example, acetate buffers have the highest buffering capacity when the pH is between 3.5 and 5.5 because the pKa of acetic acid is 4.56. In practice, buffers are prepared by adding an acid or a base to a salt solution (concentration: a few mM to a few dozen mM) of its conjugate base (e.g. sodium salt) or conjugate acid (e.g. ammonium salt).
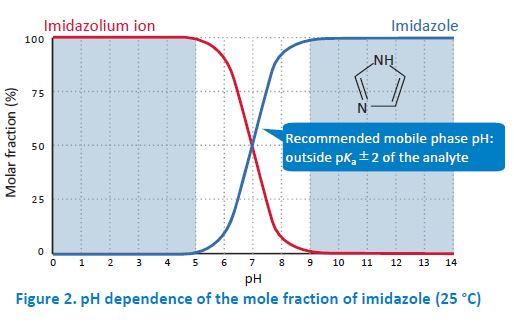
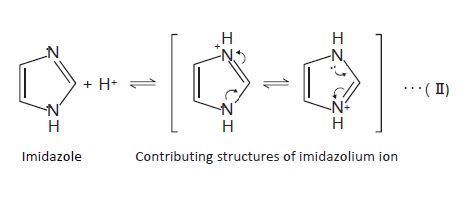
The pKa of the analyte is another important factor. If the pH of the mobile phase is around pKa of the analyte, the reproducibility of the retention time becomes low. This is because a slight pH difference between mobile phase preparations changes the degree of dissociation of the analyte. Particularly, it is recommended to set the mobile phase pH smaller than pKa−2 or higher than pKa+2. Fore example, imidazole is a basic compound with pKa of 7.01. Therefore, most of the molecules exist as an imidazolium ion when the pH is below 5, while most of them are in their non‐ionized form when the pH is above 9.
In general, the retention in reversed phase HPLC becomes low when analytes are in their ionic forms. In other words, if the pH of the mobile phase keeps the analytes in their non‐ionized form, the retention becomes relatively strong. For example, imidazole is more strongly retained in the column when the pH of the mobile phase is above 9 compared to the pH below 5 where imidazole is ionized. Base‐resistant columns such as InertSustain C18 enable analysis with such basic mobile phases. Ion‐pair reagents are used to retain ionized compounds.
Therefore, the pH has to be adjusted such that the analytes are ionized. When analyzing imidazole with an ion‐pair reagent, for example, the pH below 5 is typically employed to ionize imidazole.