2. Organic Solvents for Mobile Phases
In Reversed Phase Mode
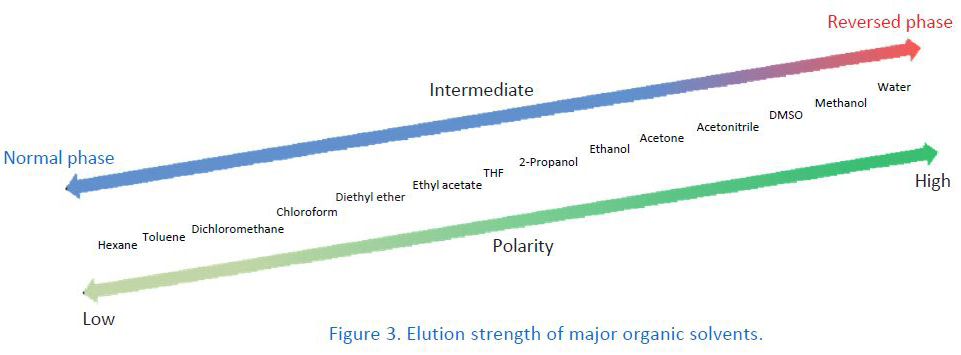
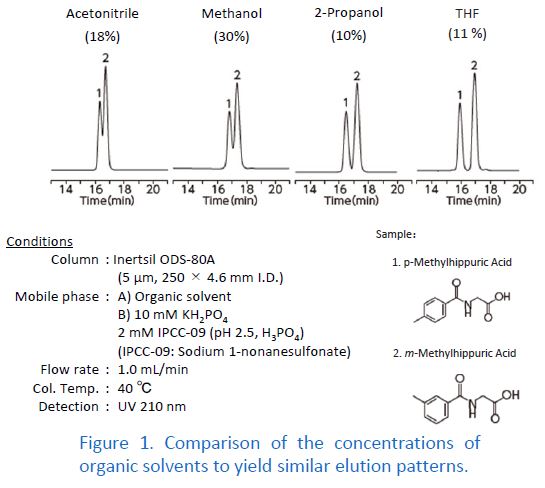
Changing the type of organic solvent in the mobile phase varies retention times in many cases. This is because the elution strength depends on the type of organic solvent. In order to keep the retention times constant among different organic solvents, the mobile phase composition has to be adjusted. When there is a change in the type of organic solvent, it is recommended to perform peak identification again because the elution order might be changed. In some cases, overlapping peaks are separated by changing the type of organic solvent.
In HILIC Mode
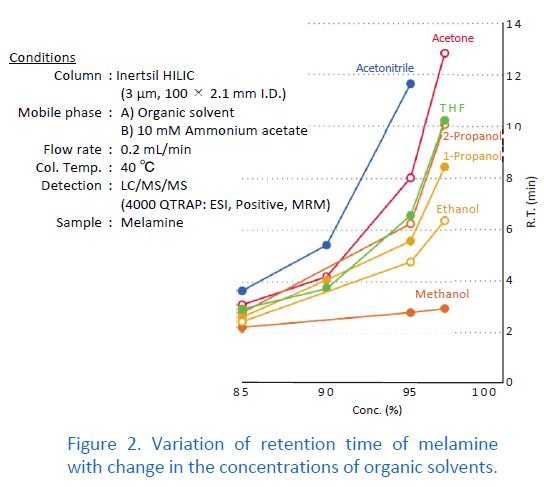
Acetonitrile is by far the most commonly used organic solvent in HILIC mode. When switching the organic solvent from acetonitrile to methanol, the retention becomes too weak in practice. Alternatives to acetonitrile in HILIC mode are acetone and THF. However, note the disadvantages of these two solvents. Acetone is unsuitable for UV detectors because of its higher UV absorption than that of acetonitrile. THF causes swelling in PEEK, which is widely used for HPLC tubing. Therefore, make sure that there are no wetting parts made of PEEK in your HPLC system when using THF.
In Normal Phase Mode
It is common to use mixtures of hexane and ethanol for the mobile phase. However, when the retention for the target compound is too weak, the ethanol concentration has to be extremely low. In this case, 2‐propanol or ethyl acetate, which is less polar solvent than ethanol, is alternatively used. The weaker elution strength of these solvents allows for a higher concentration in the mobile phase, which makes mobile phase preparation easy and improves reproducibility.